摘要:下面介绍的是18小时搞定AP化学【知识点最全总结】,备考AP化学的考生,快来看看吧。
18小时搞定AP化学【知识点最全总结】,希望对大家备考AP化学考试有帮助。
第一天> 原子, 核化学
知识点:
1)了解Dalton, Thomson, Millikan, Rutherford探索原子结构时的主要手段和成果;熟悉Bohr模型和L.de Broglie波粒二相性的理论和相关公式, 会用Heisenberg测不准原理解释原子结构。
2)能懂且会用四个量子数表示电子排步。
3)理解Pauli, Aufbau, Hund, Energy Overlay这四条电子排步原则,熟记各条反例,如Cr, Cu, Mo, Ag这些半满轨道排步。
4)能解释原/离子半径、电离能、电亲和势、电负性的周期性递变原因;熟记s/p-block、半金属元素的符号缩写和英文全拼。
5)牢记核衰变反应的定义,各类核反应释放出的微粒or射线实质;熟练配平反应式和计算半衰期。
Electron model
1. Black body radiation—undermine classical physics--Mac Plank E=hv -- photoelectric effect
2. Spectra: continuous spectrum(all color), emission lines spectra(color with special energy are on the spectra), absorption spectra (black lines, reverse of emission lines)
3. En=-Rn(1/n^2) 1/wavelength=Rh(1/(n1)^2-1/(n2)^2)
4. For one electron atoms or ions: Bohr Model (classic physics+ Rutherford model+ quantum theory)
(1)angular momentum(energy) is quantized, electron in orbit radiate no energy
(2)photon-excitization-excited state-move back-electromagnetic radiation
(3)e can exist in possible circular orbit with fixed radius
5. E=-2.18*10^-18/n^2
6. First ionization energy of H: 2.18*10^-18
7. wave and particle duality: De Broglie wavelength=h/p=h/mv=10^-10m
8. Heisenberg uncertainty principle: dx*dp>=k/4pi. It is impossible to determine position and momentum at the same time.
9. Wave mechanical model:
(1)calculate possible energy states and positions
(2) Quantum number and wave function (probability of locating an e in a region in space)
10. Quantum numbers:
(1) principle quantum number: n, determine size and energy of orbital
(2) angular momentum number: l (0——n-1) shape of orbital
(3) magnetic quantum numbers: ml (-l——l) orientation
(4) Spin quantum number: ms=+-1/2 spin
11. when Z>1 : nuclear charge is greater, e and e repulsion, penetration (cause of fraction Zeff)
Period:
1. Group1: alkali metal, group2:alkali earth metal, group5:pnictogens(stifle), group6: chalcogens (elements to make ore), group7:halogen, group8: noble gas
2. Transition metal: 3-12. Inner transition element: actinides and lanthanides
3. Metallic character decreases from Fr to F
metal / non metal
reducing agent / oxidizing agent
heat/electric conductivity no heat/electric conductivity
shiny / not shiny
hard/soft
Ductile , compressible/not ductile, compressible
delocalized e/no delocalized e
basic oxide/acidic oxide
4. Semimetal: conduct electricity and very low temperature, superconductor
5. D-block: colored compound, complex ion, many oxidation states, magnetic properties, catalysis
6. Atomic radius: increase down a group because: valence n increases, valence e further from nucleus, an extra full shell repels other e to make it bigger
Decrease across a period because: e is added to the same valence shell, shielding by inner shell is constant, Zeff increases so valence e is pulled closer
7. Radius of ions: cation is smaller and anion is bigger.
8. Electronegativity: ability of a bonded atom to attract a shared pair of e.
9. Down a group and En decreases because atoms have bigger radius. Across a period En increases because atom is smaller and Zeff increases.
10. Ionization energy: first ionizing energy: E(g)-E+(g)+e- second ionizing energy: E(g)-E2+(g)+2e-
11. Down a group IE decreases as radius increases and nuclei have less attraction of valence electron, across a period IE increases as Zeff increases, attraction increases and it is harder to pull a e away. Small trend because e is spinning paired and repulsion exist between 2e in the same orbital
12. Successive ionization energy: pick 1 element and takes away its electrons one by one, the jump of energy is orbital because: when there is less e, more attracted to nucleus, repulsion is smaller with less e, when removed a shell, it is harder to move another e as it is closer to the nucleus
13. Electron affinity: first EA: E(g)+e—E-(g)
14. Trends are not clear but basic trends are: down a group less negative as radius increases-attraction decreases-less energy released, across a period more negative as Zeff increases-attraction increases-energy released increases.
15. Group1: lowest 1stIE in periodic table, slightly negative EA, keen to give up e but weakly to accept e
16. Group7: high1stIE, high negative EA, lose e with difficult but gain easily
17. Group8: very high 1stIE, slightly positive EA, tend not to lose or gain e easily.
题-Multiple Choice (MC)选自 Barron’s 2009 ed., Free Response (FR)选自历年真题:
MC: Chp.1 –Question (Q#) 10, 12, 13, 15, 22; Chp.2 -Q6, 9, 13, 14; Chp.3 –Q1-3, 5, 7, 9。
FR: 2010年 Q6 (a-c), 2007年Form B Q2、Q7 ( a-c (i) ),2006年Form B Q7 (a, c, d)。
第二天> 分子内的键和分子间的力,一丢丢化学运算题
知识点:
1) 能判别离子、金属、共价键,以及分析前两者强弱。
2)会画共价化合物的Lewis dot structure ,20秒一个。能从重叠方式不同解释sigma和pi键的区别,知道键级、键能、键长的联系。
3)能用VBT解释共价键实质,用HOT解释中心原子如何杂化,用VSEPR解释成键电子/孤对电子间如何排斥并占据空间。
4)能分析出且熟记成键电子数 + 孤对电子数小于等于六时的各种空间构型,包括中心原子的轨道杂化方式、几何体名称、键角、分子极性。
5)根据极性不同判断出三类分子间作用力,知道其强弱各自受哪些因素影响,以及它们的相对强弱。
6)掌握化合价配平法,半分钟一个式子;关于mole运算、经验/分子式、净离子方程式...这些初中就开始练的题,怎么还好意思错呢。
Bond
1. Reason of bond: electrostatics, lower their energy
2. Bond: covalent(non-metal and non-metal), ionic(non-metal and metal), metallic(metal and metal)
3. Lewis theory: octet rule. Every atom tens to have 8 e. discovered by noble gas
4. Lattice energy: a measure of strength of attraction between ions Na(g)+ Cl(g)- NaCl(s) unit is KJ/mol. Measured in born-haber cycle(chemical way) and born-mayer cycle(LE=k(Z+*Z-)/r)
Ionic solid:
1. giant 3-D lattice
2. Hydration energy: energy used to make ions in water completely surround by a shell of water molecule. When LE
3. High melting point and boiling point: it is hard to make ions vibrate so harder to pull them apart.
4. Conduct electricity: when molten or dissolved in water because ions can mobile
5. Brittle: when hit, cation and cation (anion and anion) will move and repel each other.
Metallic bonding
1. low melting point and high boiling point: it is easy to pull metal atoms apart but it is hard to completely separate an atom
2. good thermo and electric conductivity: mobile e-
3. luster
4. Malleable and ductile: forces of attraction are not broken. Ion slide over each other but still held together
5. melting point increases when Z increases: more e- and less radius
Covalent bond
1. bond length and strength: long the bond, weaker it is
2. bond energy is measured in KJ/mol
3. poor conduction of electricity: no delocalized e- (except graphite)
4. melting point and boiling point: - giant lattice: high because hard to break bond -molecular solid: low because IMF is weak
5. diamond: -hard: 3D interlocking of covalent bond -not conduct electricity: all valence e is used in bond
6. Graphite: -conduct electricity: delocalized e -soft weak: LDF between layers are easy to overcome by force.
Coordination compound
1. Contains complex ion and counter ion. Ex: [Co(NH3)6]3+(complex ion) + Cl-(counter ion)
2. ligand: provide both electron for covalent bond so must have lone pairs. Are around transition metal in complex ion.
3. name: name cation first, include oxidation state of cation, then name anion ex: hexaamminecobalt(iii) chloride
4. Formation reason: d orbital of metal is empty and overlap with electron orbital of ligand.
5. dative bond: a covalent bond In which the pair of e- is supplied by one of the 2 balanced atoms (Lewis base provides e- while Lewis acid do not)
Lewis structure
1. Decide Lewis structure: -formula –central atom(one with lowest EN except H) –count e- -draw skeletal structure –add e-
2. electron deficient compound may violent octet rule ex: BF3
3. Formal charge: the electric charge an atom would have if all bonding electrons were shared equally with its bonded neighbor. Normally between -1 and +1
4. resonance is actually delocalized pi-bonds
5. Bond order: the bonds between two atoms, resonance are calculated by taking average
Molecular shape and inter molecular force
1. VSEPR theory: valence e- pairs repelling to minimize energy of molecule or polyatomic ion. Valence e- maximizes distance apart and determine the shape of molecules.
2. geometry shape:
3. all lone pairs are not include in the geometry shape
4. hybridization: (1)sp linear BeH2 (2) sp2 trigonal planar BF3 (3) sp3 tetrahedral (4)sp3d trigonal bipyramid PCl5, XeF2 (5)sp3d2 octahedral SF6, XeF4O, XeF4
5. molecular orbital theory: explains resonance like benzene
6. polarity:
1. non polar: no permanent polarity, no dipole
2. Polar: delta EN not zeros
7. Intermolecular forces:
(1)LDF electron fast moving causes temporary dipole. Down a group stronger because of more electrons (more polarizable) and bigger(less attraction of electron), more surface area contact stronger
(2) Hydrogen bond: because of (a)highly EN FONCl(b) no inner shells in H(c) small size of FONCl
(3) Ion dipole: ion + polar mole
(4) dipole-dipole
attraction forces
ion present
no ions
ions only
polar only
non polar only
ionic bond
dipole-dipole
LDF
8. Like dissolves like: polar-polar force to overcome lattice energy, non polar- non polar because of entropy and enthalpy.
题:
MC: Chp.5 – Q10, 11, 13, 14; Chp.6 – Q5, 14, 17, 18, 20
FR: 2014年Form B Q5,2012年 Q4, 2006年Q7 (a-b), 。
第三天:物质的气、液态与变相
知识点:
1) 灵活应用气体分子的总/平均动能计算公式,以及可爱的Graham’s Law, 知道由PV=nRT推出来的系列公式所对应的不同恒定量假设。
2)牢记理想气体的三个假设及其宏观影响、Van der Waal’s Eq.里a、b的对应含义,会应用气体分压概念进行各种定量运算。
3)能找出三相图、加热/冷却曲线图里的各种特殊点或线,尤其特殊的水。
4)了解溶液和电解质的定义,牢记浓度的两种表达方式、四个colligative properties的含义和定量计算。
5)牢记可溶物和难溶物各自的溶解规律及溶解度表示,理解Ksp和同离子效应的定量运算。
Gases
1. Pressure:1.01*10^5Pa=1atm=760mmHg
2. Temperature: K=C+273.15
3. Boyle’s law: PV=k(n,T is kept constant) Charle’s law: V=kT(P,n is kept constant) Avogadro’s law: V=kn(P,T is kept constant)
4. Kinetic molecular theory: assume(1)volume is neglectable (2) no IMF (3)elastic collision (4) average KE=T
5. Peak of number of molecule vs speed graph is approximately average KE
6. m1/m2=average v2^2/average v1^2 v1/v2=√m2/m1
7.Graham’s law of diffusion :average KE=1.5RT=0.5MV^2(for 1 mol) average v=√3RT/Mr (root-mean-square speed)
8. Real gas: tend to act like ideal gas when: T is high and P is low
9. (P+an^2/v(volume)^2)(V-bn)=nRT
题:
MC: Chp.7 – Q8, 19, 20; Chp.8 – Q3, 13-16; Chp.9 – Q14, 17, 18, 20, 21。
FR: 2014年Q1、Q4 (a-b), 2013年Q1, 2012年 Q2 (a-e), 2011年 Form B Q2 (a-c), 2010年Q1。
第四天:反应的速率与平衡
知识点:
1)熟练运用实验法找出任一反应的Rate Law, 任一反应物的rxn order,k的数值和单位,熟记0,1,2级反应的对应的公式和各种表达图像,理解线性图像的特殊意义。
2)应运碰撞理论和Arrhenius Eq.分析反应速率;牢记激活能、反应物浓度、温度、压强与反应速率的正负相关性。
3)理解基元反应的molecularity概念,会用多步反应中slow-determining step的反应机制替代总反应机制 。
4)牢记平衡常数的定义式,熟练掌握8种特殊情况下的Keq计算法。
5)熟练应用Le Chatelier’s Law, 分分钟解释Haber process。
Reaction Rate
1. collision theory: reaction is caused by collision with right orientation and enough Ea
2. -surface are increases: more probable of successful collision -temperature increases: more molecules with energy more than Ea
3. Archenius k=Ae^(-Ea/RT) A is pre-exponential constant R=8.314J/mol*K
4. rate= k[A]
5. Ea= Rln(k2/k1)/[(1/t1)-(1/t2)]
6. Reaction profile: for a chemical reaction Ea is fixed. Transition state is the state with highest PE, catalyst will decrease Ea without affecting delta H
7. r= k[A]^x*[B]^y*[C]^z x,y,z are the orders with respect to A,B,C
8. Initial rate method: (a)vary [a] and keep others constant and then (b) vary [B] and keep others constant. Log r= log k +x*log[A]i
9. mechanism: many simples steps together to form a reaction
10. intermediate: produced in one step and consumed in another
11. one can check if his mechanism is correct by finding intermediates.
Nuclear chemistry
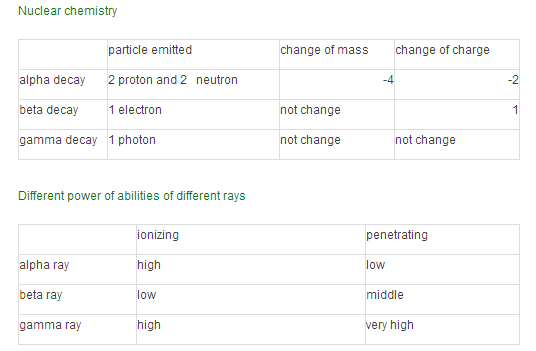
Biological effect:
(1) alpha: harmless, cannot penetrate skin
(2) Beta: high, can harm bones, organs
(3) Gamma: high, will ionize molecules can cause them to repeal each other
6. Fission and fusion:
(1) fission: one nuclear becomes more than one particle, like U235
(2) Fusion: two or more nuclei combine to become one nuclear
题:
MC: Chp.11-Q6, 8, 13,17, 20, 22; Chp.10 –Q7, 9,13,17, 19
FR: 2012年 Q3 (d-f), 2011年Q6 (c-d), 2010年Form B Q3 (c-f)。
第五天: 热力学,酸碱和氧化还原反应
知识点:
1)ΔS, ΔH, ΔG的含义和在标准非标准状态的正负值/定量运算,尤其是ΔH,那么多算法,你懂的。
2)关于热容、比热容、ΔG =ΔH - TΔS这些公式的运算和正负值含义。
3)熟练掌握酸碱的三种分类法,包括秒找共轭对,强/弱酸碱的判定,盐的弱酸/碱性判定,缓冲溶液的构成和作用原理。
4)能结合应用氧化数的增减和LEO & GER口诀,随手拆出两个半反应方程式,知道金属活动性和氧化电动势正相关。
5)理解酸碱滴定曲线的图像变化,从而适当选择指示剂;了解两种电池的运作原理;能够熟练判断电池两极,及其装置用途和实验现象。
题 (这天以知识点为主,题不多,明天集中刷定量计算):
MC: Chp. 12-Q9-11, 12, 13, 14
FR: 2014年 Q6 (c), 2013年Q3, 2012年Q3
第六天:酸碱和电化学的计算
计算出题点:
1)强/弱酸、强/弱碱、非中性盐、缓冲溶液、滴定过程中某一时刻的pH, pOH, Ka, Kb,某离子浓度的运算,全都要会,没商量。
2)会算转移电子数,两种电池的标准/非标准电池电势;用法拉第公式计算转移电量和电流;用Nernst Eq.把ΔG,Keq, Q,和Ecell联立解决,包括Q=K或者T=298K这些特殊情况下的简化公式。
题:下方高能预警!
MC: Chp.14-Q4, 5, 9, 22, 24; Chp.13-Q12, 13, 16, 18, 22
FR: 酸碱> 2014年Q2, 2012年Q1, 2011年Form B Q1, 2011年Q1, 2010年Form B Q5。氧化还原> 2014年Q3, 2013年Q2, 2012年Q6, 2010年Form B Q2。
第八天:一套半选择题
从第119页开始,26道最新版选择样题,限时40分钟,认真做吧。
一套来源:自选限时完成,做完务必解决所有疑惑。
第九天:2008年的一整套真题
之前建议的每日习题完美避开了2008年,我会乱说吗?而且这套题是世面上难得的有全部答案解析的整套真题,特别赞!
最后,不是祝愿,是相信,所有努力过的同学们都能拿到五分,然后开开心心看复联2首映!
以上整理的就是18小时搞定AP化学【知识点最全总结】,希望考生们可以认真学习。如果想了解最新AP考试资讯,请关注小马过河AP频道,关于本文有任何疑问,请和小马在线专家联系。
(AP课程包括:AP生物、AP美国历史、AP计算机、AP心理学、AP欧洲史、AP化学、AP英美文学、AP微积分、AP物理、AP化学等。AP师资团队全部是由顶尖名校毕业、且AP教学时长在3000个小时以上的教师亲授课程。授课方式:一对一全程名师督导备考陪同,量身定制互动直播授课,点题讲题破题一步到位,反复实战演练,助力AP考生备考冲刺5分。)
咨询课程顾问,免费领取全套AP考试指南和真题学习资料。

